Abviris Deutschland GmbH
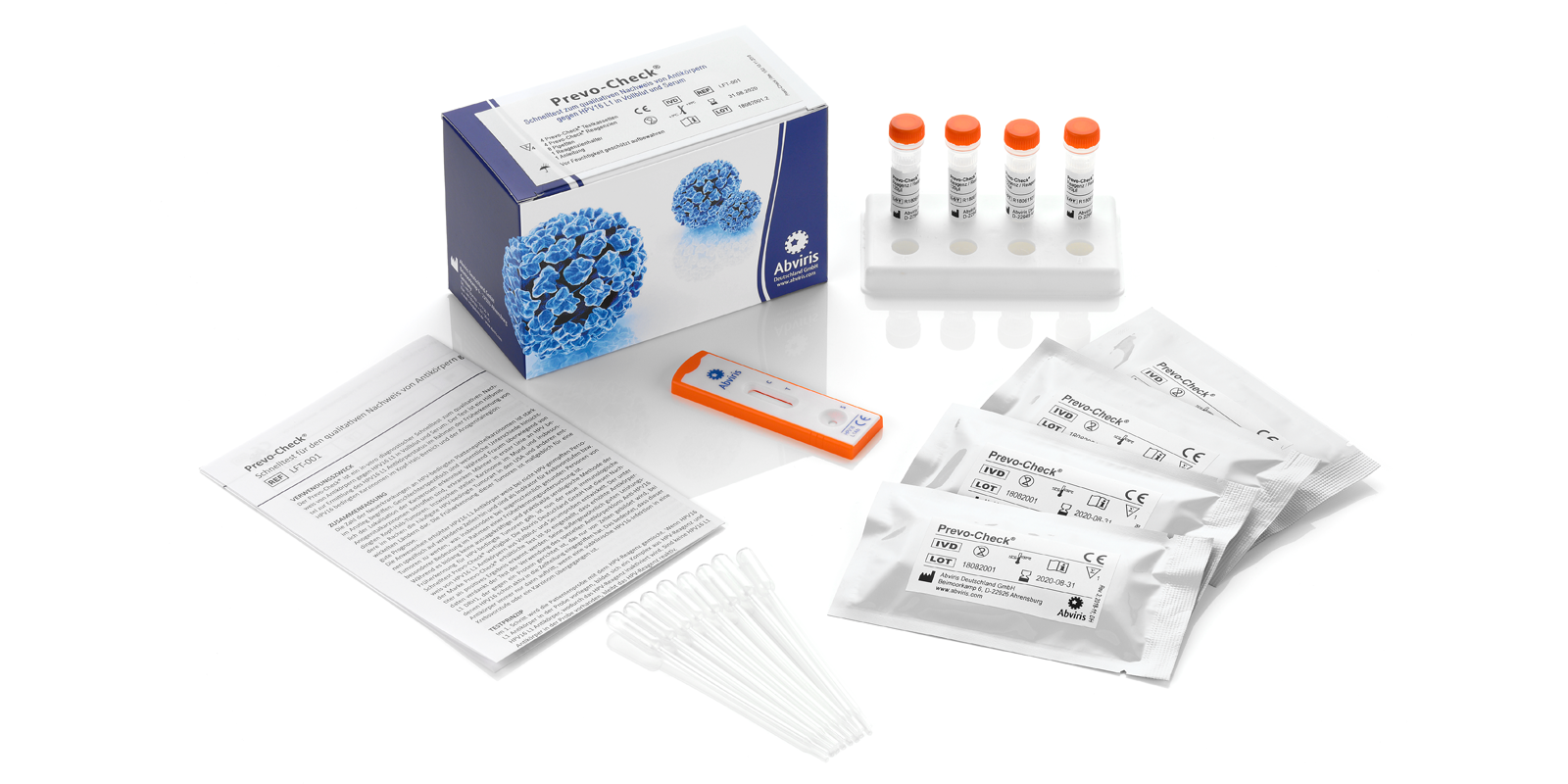
Prevo-Check®
Abviris is tackling the global increase in cancers caused by human papillomavirus. It aims to increase the chances of recovery for cancer patients and help hospitals around the world to detect HPV‐ induced cancers in the head, neck and anogenital region earlier and consequently treat them more successfully.
There had been no practical and reliable serological method for early detection of HPV 16‐induced cancers until Prevo‐Check®, the new rapid immunoassay now available as an IVD product.
Prevo-Check®
Product Code: LFT-001
CE IVD, for professional use only, 4 tests included
-
For early detection of HPV 16‐induced cancers
-
High clinical specificity of ≥ 99.3%, clinical sensitivity ≥ 90%
-
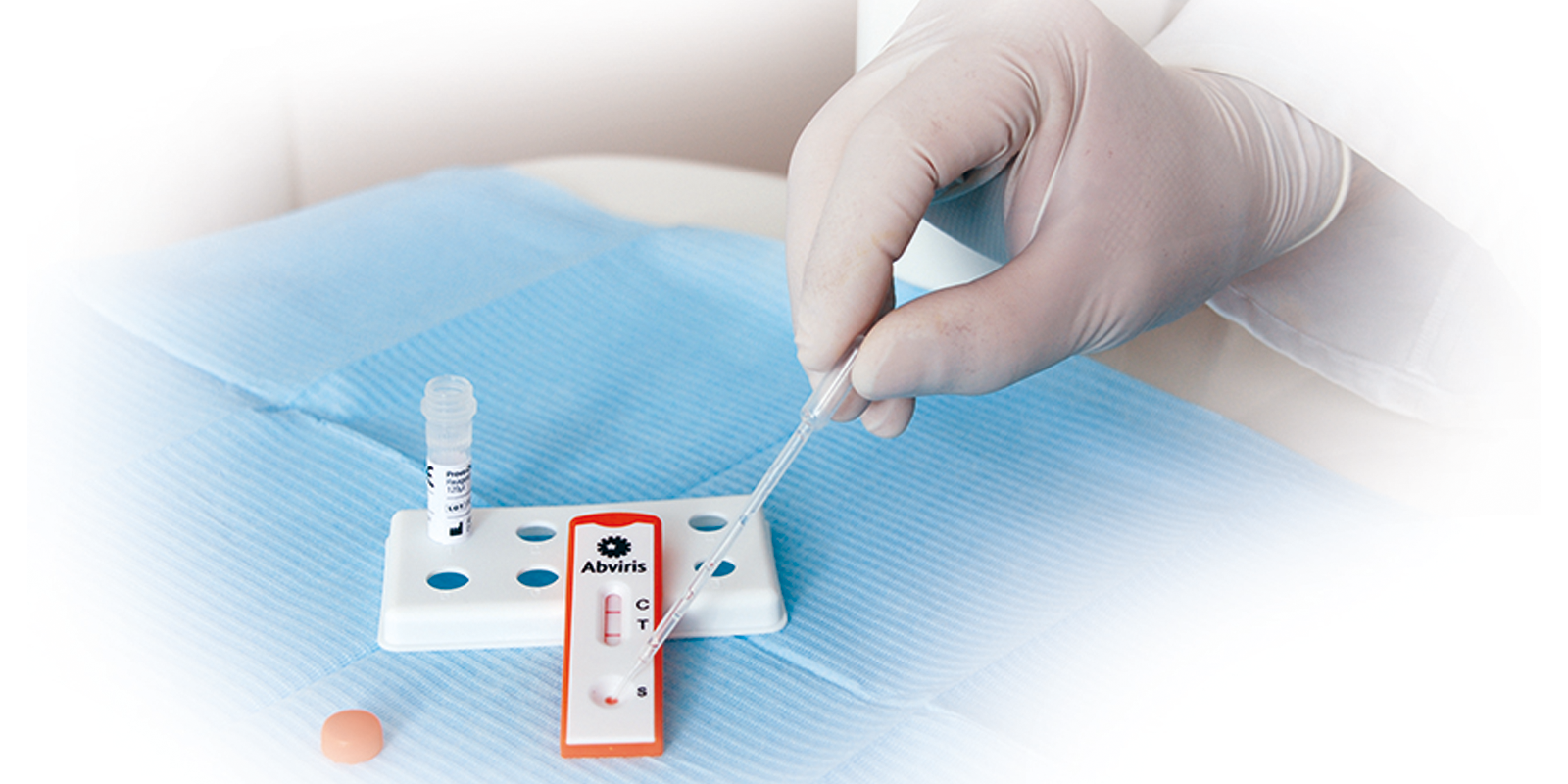
Easy to use with just one drop of blood or serum
-
Results in minutes
From a diagnostic perspective, it is not the common HPV infections that are important to detect, but rather HPV‐induced diseases.
In people who have not been vaccinated against HPV, an increase in anti‐HPV 16 L1 antibodies is a specific indicator of cell changes and should be interpreted as a sign of precancerous lesions or tumours. This is very important in screening, particularly of people who appear healthy.
This test owes its exceptional performance data (clinical specificity ≥ 99.3%, clinical sensitivity ≥ 90%) to the use of specific anti‐HPV 16 L1 antibodies, which are directed against a protein produced only by cells in which HPV 16 has already actively interfered with cell division. This means that these antibodies are only present when a subclinical HPV 16 infection has progressed to a precancerous lesion or carcinoma.
MHM-Diagnostics
Franz-Beiske-Weg 19
48167 Münster
Medical Devices Vertrieb und Medizinproduktberatung Health Care Management
-
Telefon / Phone+49 (0)251 67498282
-
Mobile+49(0)171 7721083
-
E-Mail
This email address is being protected from spambots. You need JavaScript enabled to view it. -
Fax+49(0)251 67498283
Please send us requests for more literature, information, brochures, test orders or quotes by e‐mail, stating your name, organisation, department, telephone number and e‐mail address.